Kittel, 5
In solids, the heat capacity is defined as
where
In a previous lecture we evaluated the contribution of the electrons to the heat capacity, getting that their contribution is minimal. What we want to do now is to calculate the contribution of the phonons to the heat capacity (
where
The shape of
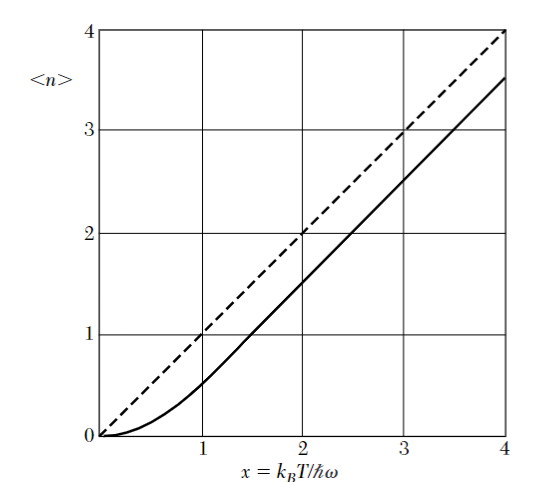
From equation
this means that the number of states between
this consideration allows us to rewrite
which can now be derived to obtain the heat capacity:
To further expand the integral we need to calculate
Phononic density of states
Debye model
My guess
to calculate Cv we still need to find the dispersion relation and thus we approximate it in different ways, one of which is the debye model?
In the Debye approximation we assume that the dispersion relation is linear:
where
With these assumptions the DOS from above becomes
If we consider a monoatomic crystal with
We will have a maximum allowed frequency (cutoff frequency) called Debye frequencytodo why?
Its values can be obtained like thistodo what is this?
From this we can also get the maximum allowed wavelength:
We can now proceed to calculate the energy
Supposing that all polarization have the same velocity (todo what does velocity have to do with this?) we get
If we define
Finding the heat capacity
We can finally derivate the total energy we just found to get the heat capacity:
In the second step above we used the fact that
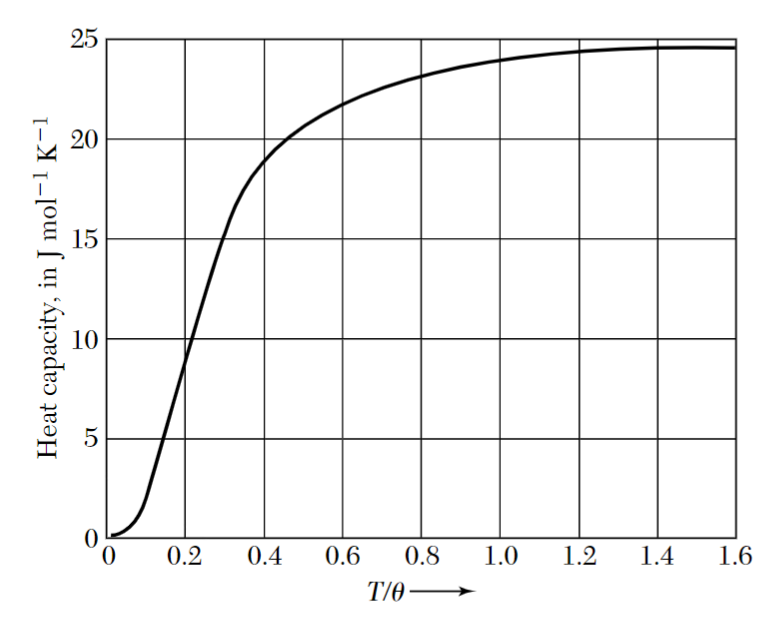
The plot above shows that the Debye heat capacity approaches a constant value equal to
Behaviour at low temperatures
In a previous lecture, we saw that the contribution to the heat capacity at low temperature given by electrons is proportional to
At very low temperatures (
(the calculation is omitted, the important thing is that the integral doesn’t depend on
This result can be interpreted in the following way:
At temperature
