Solid state physics
Solid state physics is the study of the state of the matter in which a large number of atoms (
We will not deal with amorphous materials.
Single atoms
In single atoms electrons live in radially symmetric potentials (orbitals) indicated by a number and a letter, such as 1s, 2s, 2p, etc.
The numbers represent the principal quantum number
Multiple atoms
If multiple single atoms are gradually brought closer to each other, we have an interaction between different orbitals which causes the splitting of energetic levels. This is, in turn, associated with the formation of energy bands.
todo add plot and explain
Types of bonding
- Covalent: the electrons are shared only between neighbouring atoms and the bond is typically directional.
- Metallic: the electrons are shared over many atoms and the bond is typically non directional.
- Ionic: the electrons are transferred from one atom to another because it is energetically favourable to do so, even if the overlapping is limited.
We also have weaker types of bonding:
- Hydrogen: similar to ionic bonding but without the actual donation of electrons. We have the formation of partially charged regions.
- Van Der Waals:todo
Crystals and lattices
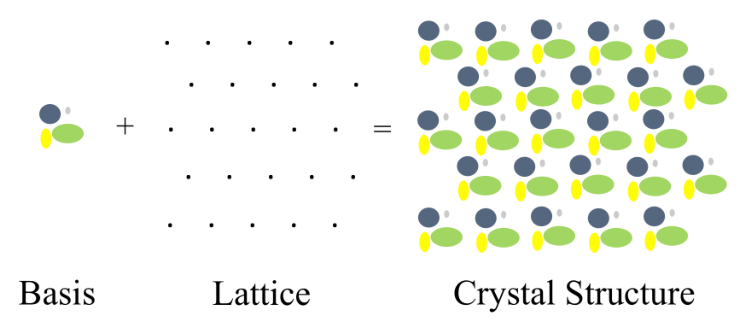
An ideal crystal has an infinite number of repetitions in 3 dimensions of the same structural unit.
The crystalline structure is composed by the lattice and the base. The lattice describes the structural spatial unit and the base represents the real position of the atoms in the lattice.
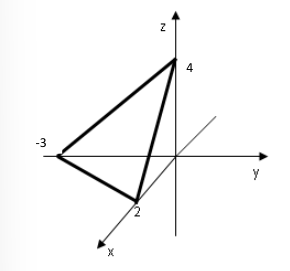
Since we are dealing with a periodic structure, what we “see” from position
The parallelepiped with edges
The primitive cell is the unit cells with minimum volume.
Since the choice of the vector
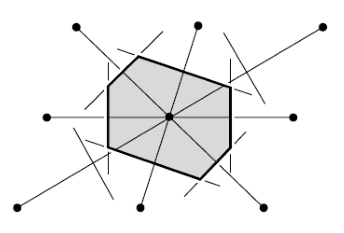
Wigner-Seitz cell
Wigner-Seitz cell is a special choice of primitive unit cell: region of points closer to a given lattice point than to any other.
How to draw it in 2D:
- Find the point for which you want to draw the cell.
- Draw lines that connect it to all its nearest neighbours.
- For each of these lines draw a perpendicular line(
) that passes through its mid point. - The area delimited by the lines (
) is the Wigner-Seitz cell.
Bravais lattice
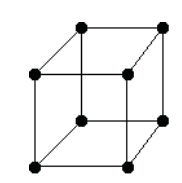
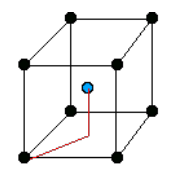
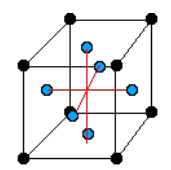
There are only 14 unique lattices possible in 3 dimensions called Bravais lattice. We are mostly interested in studying the cubic lattice which comes in three forms:
Simple Cubic Body Centered Cubic (BCC) Face Centered Cubic (FCC) the blue atom is in the center of the cube the blue atoms are on the faces of the main cube Each lattice has a specific lattice parameter
Link to originalwhich represents the length of the cube side. It should be noted that BCC and FCC are not primitive because they contain more than one point (the primitive cell wouldn’t show the cubic symmetry).
Link to original
Miller indexes
Miller indices
From what we will see later, the same material, with the same lattice structure can behave very differently depending on how it is cut (for thin films we are interested in the surface properties). To be able to describe different surfaces we can use Miller indices to identify the cut plane. The method to find those indices is as follows:
- Find the points where the cutting plane intercepts the
axes, in this case - Write the reciprocal of the numbers
- Reduce the list to the smallest integer
- Usually negative numbers are represented with a bar, the Miller index of this plane is
. Link to original